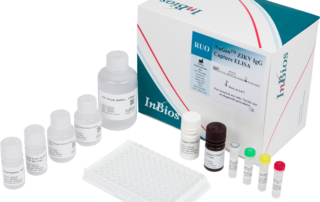
ZIKV Detect™ IgM 2.0 Capture ELISA
FDA Cleared for In Vitro Diagnostic Use
The ZIKV Detect 2.0 IgM Capture ELISA is intended for the qualitative detection of Zika virus IgM antibodies in human sera for the presumptive clinical laboratory diagnosis of Zika virus infection.

Resources
- 1st Commercial serology kit to receive FDA Marketing Authorization
CE Marked
Differentiates Zika IgM positive patients from those infected with other flaviviruses such as West Nile or Dengue
Relative Sensitivity: >90%
Relative Specificity: >96%
Results in 4 hours
Tests up to 28 unknown specimens
| Catalog No. | ZKM2-1 |
| Format | Immunocapture |
| Quantity/Kit | 96 wells/plate |
| Incubation Time | 60+60+30+30+20+1 |
| Sample Type | Serum |
| Storage | 2-8°C |
| Shelf Life | 9 months |
For more information, download instructions for use via the “Downloads” tab.
Related Products
Scrub Typhus Detect™ IgM ELISAinfo@inbios.com2023-10-11T16:08:17+00:00
Scrub Typhus Detect™ IgM ELISA
Scrub Typhus Detect™ IgG ELISAinfo@inbios.com2023-10-31T15:16:56+00:00
Scrub Typhus Detect™ IgG ELISA
West Nile Detect™ IgM Capture ELISAinfo@inbios.com2023-10-11T21:52:46+00:00