CL Detect™ Rapid Test for Cutaneous Leishmaniasis
FDA Cleared for In Vitro Diagnostic Use
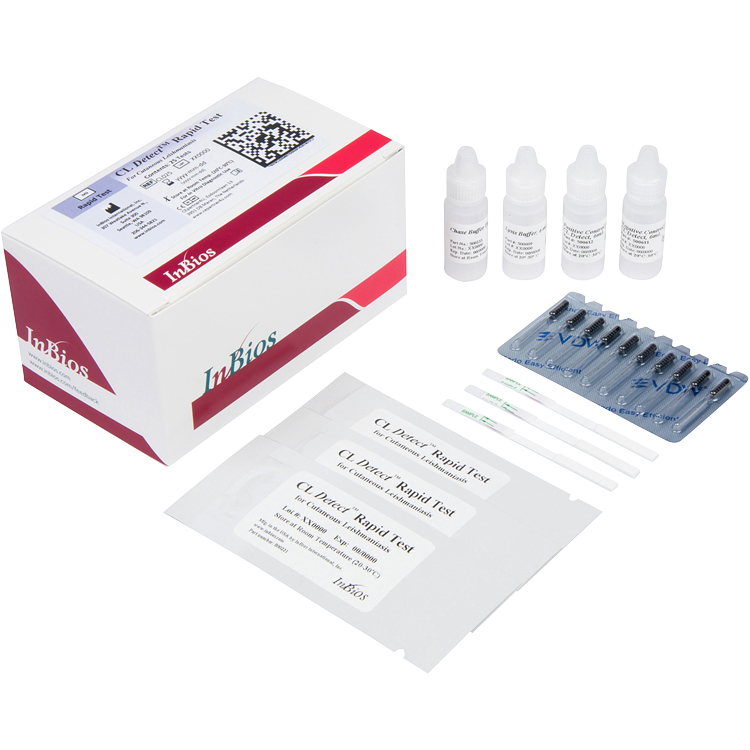
The CL Detect™ Rapid Test is a qualitative, in vitro immunochromatographic assay for the rapid detection of Leishmania species antigen in ulcerative skin lesions.

Resources
- US FDA Cleared
- CE Marked
Relative Specificity in non-endemic population: 96%
Relative Sensitivity and Specificity in endemic population: 100% and 84% respectively
Kit includes 25 tests, controls, buffers and sterile dental broaches
| Catalog No. | CL025 |
| Format | ICT (strip) |
| Quantity/Kit | 25 tests |
| Time to Result | 20 minutes |
| Sample Type | Skin lesions |
| Storage | Room Temperature (20°C-30°C) |
| Shelf Life | 24 months |
For more information, download the instructions for use via the “Downloads” tab.
Related Products
Scrub Typhus Detect™ IgM Rapid Testinfo@inbios.com2023-10-11T21:29:21+00:00
Scrub Typhus Detect™ IgM Rapid Test
Scrub Typhus Detect™ IgG Rapid Testinfo@inbios.com2023-10-11T21:30:28+00:00
Scrub Typhus Detect™ IgG Rapid Test
CL Detect™ Rapid Test for Cutaneous Leishmaniasisinfo@inbios.com2024-01-08T16:50:55+00:00
CL Detect™ Rapid Test for Cutaneous Leishmaniasis
Kalazar Detect™ Rapid Test for Visceral Leishmaniasisinfo@inbios.com2023-10-11T21:32:11+00:00
Kalazar Detect™ Rapid Test for Visceral Leishmaniasis
Chagas Detect™ Plus Rapid Testinfo@inbios.com2024-01-19T23:43:01+00:00
Chagas Detect™ Plus Rapid Test
Active Melioidosis Detect™ Rapid Testinfo@inbios.com2023-10-10T19:04:25+00:00
Active Melioidosis Detect™ Rapid Test
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-03-06T18:31:08+00:00
Active Anthrax Detect™ Plus Rapid Test
Chagas Detect™ Plus Rapid Testinfo@inbios.com2024-01-19T23:43:14+00:00
Chagas Detect™ Plus Rapid Test
Active Anthrax Detect™ Plus Rapid Testinfo@inbios.com2024-03-06T18:31:05+00:00